Gene Therapy
Overview
In amyotrophic lateral sclerosis (ALS), gene therapy may help if it can deliver a beneficial protein to salvage dying nerve cells. Gene therapy can be simply a means to boost on site production of a helpful factor, at places where nerve cells are in trouble. Researchers can disarm many types of viruses, and put in the genetic instructions to make therapeutic protein. The redesigned viruses are called vectors. They are simply carriers for therapeutic genes. Knowledge of the SOD1 mutations linked to some forms of ALS has produced a vast body of evidence, pointing to a general strategy for the disease that might be successfully implemented through gene therapy.
What is gene therapy?
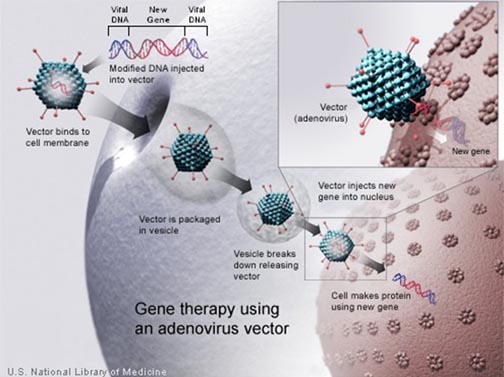
Gene therapy is the use of genetic instructions to produce a protein to treat a disorder or deficiency. It can aid in a disease even if the therapy is not directly targeting a gene defect that causes the disease. In amyotrophic lateral sclerosis (ALS), gene therapy may help if it can deliver a beneficial protein, to salvage dying nerve cells. The gene therapy simply is a means to boost on site production of a trophic (growth enhancing) factor, at places where nerve cells are in trouble.
Genes are the molecules in all cells of our bodies that carry the instructions to make all of the materials that comprise the body. In the 1950s, scientists determined that genes code precisely for proteins, with a sequence that specifies the order of the building blocks of proteins, the amino acids. Each gene corresponds to a protein. Each base in a gene codes for an amino acid. The order of bases in a gene produces the ordered chain of amino acids that produce a working protein.
At the turn of the current century, scientists determined, in rough draft form, the sequence of all of the human genes. By this time, they also knew how to create a gene construct, and move that construct into cells, to get the cells to make the corresponding protein.
In some diseases, researchers already know that a defective gene is not able to work. They have the potential means to cure the disease, by replacing the defective gene with a correct, working copy. In ALS, only a few percent of patients have a known gene defect. For the rest, it may be one undiscovered gene that is the problem, or it may be several. But gene therapy can still be designed to aid patients with ALS by providing supportive proteins for nerve cells.
Vectors deliver genes
Genes normally reside in the nucleus, the core of a cell, separated from the surrounding materials by a membrane. The chromosomes are the structures within the cell nucleus that contain the DNA that comprises the genes. It is very challenging to get a gene made in the lab to cross both the outer envelope of a cell, and the nuclear membrane as well, to reach the chromosomes.
Scientists studying viruses have discovered nature's own solution to the problem of moving genes. Viruses are essentially genes that have evolved to hijack cells, instead of forming cells for themselves. So viruses have strategies to enter cells and take over the protein production process, to produce instead, the virus. Researchers have figured out how to use viruses as Trojan horses, to bring in genes that can then carry out genetic repairs, replacing defective DNA.
For many viruses, researchers can disarm the genes responsible for the damaging properties and put in, instead, genetic instructions to make therapeutic proteins. These viruses, redesigned by researchers, are called vectors. They are simply a means to smuggle in therapeutic genes.
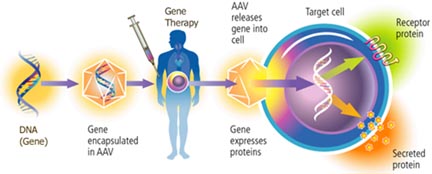
Why is gene therapy still experimental?
Investigators must demonstrate that the viral vector is not going to revert back to an infectious form, or cause any side effects.
Genes normally are read out only when a protein is needed, and operate under feedback control. If adequate amounts of protein are already there, then the gene is turned off. Scientists have to be able to devise the proper switch elements to accompany a therapeutic gene, to make sure that a gene is expressed in proper amounts, and only in an intended target tissue.
To gauge adequate delivery of the vector, and sufficient levels of gene expression, researchers have to have animal models that reflect the key manifestations of a human disease. Despite preclinical work with animals, it is not always possible to extrapolate to patients.
A patient's immune system may mount an attack on viral vectors (after all, that is what the immune system is primed to do), or on the newly introduced therapeutic gene product.
What are challenges for gene therapy in ALS?
In ALS, a few percent of patients have a known defect in a gene. This genetic mistake, in the gene coding for the SOD1 protein, produces disease no different from any other form of ALS, inherited or not. Gene therapy might be designed for a particular SOD1 defect, but that therapy may or may not work for other ALS patients.
What is encouraging is that the knowledge of the SOD1 mutations has produced a vast body of evidence for what does go wrong in other cases of ALS. And that evidence is pointing to a general strategy that might be successfully implemented through gene therapy.
Contributions of other cells in ALS
ALS research has produced the notion that the neighborhood surrounding the motor neurons can be nurturing or detrimental to these crucial cells affected in ALS. Even if a neuron carries a mutated SOD1 gene, that nerve cell can survive if neighboring support cells, the glia, have the normal gene (see section on Cell Targets).
Glial cells surround neurons. Some glial functions produce the hallmarks of damage in the nervous system, either inflammation or scarring. Other glial actions are protective, for instance, sweeping away excess excitatory signal molecules before they can do damage. Gene therapy in ALS may be able to target the glial cells as well as neurons, to produce positive effects.
Boosting helpful trophic factors
Studies in animal models of ALS show increased survival after treatment with trophic factors (see section on trophic factors), small proteins that support the growth and metabolic activities of nerve cells, most recently with IGF-1 and also after vascular endothelial growth factor (VEGF). However, clinical trials of trophic factors in ALS patients have been disappointing. The challenge of delivering these proteins to the site of damage is likely the underlying cause of the failures in the clinic.
Gene therapy may be the way to provide a steady supply of trophic factors to neurons damaged in ALS, directly at the place where the damage exists. ALSA is supporting various avenues of research that seek to implement gene therapies to deliver trophic factors, and is encouraging entry into clinical trials as quickly as possible.
Recursos en español disponibles. Por favor llame al (212) 619-1400. Gracias.
42 Broadway, Suite 1724, New York, NY 10004 | (212) 619-1400
©2019 The ALS Association Greater New York Chapter. All rights reserved.
DONATE